This method determines soluble plus exchangeable (or adsorbed) sulfur (SKCl), calcium (CaKCl) and magnesium (MgKCl) in the 1 M KCl soil suspension (1:40) from Method 20B1. While SKCl can be determined by several analytical finishes, ICPAES is preferred, as all three analytes can be rapidly determined in the same extract. Both CaKCl and MgKCl from this method have additional value when compared to Ca and Mg in peroxide digests (CaP and MgP) obtained from Method 20E1. The comparisons (differences) indicate the extent of release via acid dissolution of Ca and/or Mg carbonate/s, oxide/s or hydroxide minerals. Thus, an estimate of the acid neutralising capacity of the soil is achieved (see Method 20I1).
≈ 1 M Hydrochloric Acid
Add about 100 mL HCl to about 400 mL deionised water, mix, cool and dilute to 1 L.
Primary Mixed Standard for ICPAES analysis of Ca, Mg and S
1 L contains 1000, 200 and 1000 mg of Ca, Mg and S, respectively.
Prepare from separate commercial standard concentrates each of 10 000 mg/L of Ca, Mg and S or dry calcium carbonate (CaCO3, Primary Standard grade) by heating at 110°C to constant weight. Also dry magnesium oxide (MgO, heavy) by heating in an electric muffle furnace at 600 to 700°C for 2 h, and dry anhydrous sodium sulfate (Na2SO4) at 105°C for 4 h. Cool and store each of the chemicals separately in desiccators without desiccant.
Weigh 2.4973 g CaCO3, 0.3316 g MgO and 4.430 g Na2SO4 and wash each into a 1.0 L conical flask with about 50 mL deionised water. Add 240 mL ≈1 M HCl and boil until all CO2 is expelled. Cover and allow to cool, then transfer quantitatively to a 1.0 L volumetric flask. Dilute to volume with CO2 free (boiled) deionised water and mix well. Transfer to a clean plastic bottle. Should MgO not assay at 100% purity, adjust the weight according to the assay obtained.
Combined Ca, Mg and S Secondary Standard
1 L contains 100.0, 20.0 and 100.0 mg of Ca, Mg and S, respectively.
Accurately dilute 100 mL of Mixed Ca, Mg and S Primary Standard to 1.0 L in a volumetric flask using deionised water.
Combined Working Standards for ICPAES analysis of Ca, Mg and S
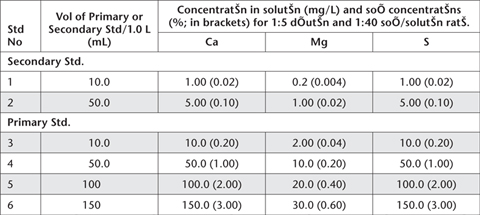
Prepare by diluting specified volumes (mL) of Primary and Secondary Standards as per Table 20.4. To each working standard (1.0 L volume) add 14.9 g of analytical grade KCl to match the matrix of the samples, prior to making to volume. Actual concentrations of Ca, Mg and S in these solutions are tabulated (Table 20.4), as are direct soil concentrations (%), based on a 1:40 soil/solution ratio and a 1:5 dilution.
Proceed from the end of the titration step in Method 20B1. Quantitatively transfer contents of the titration vessels to tared or weighed beakers using deionised water. Add deionised water to the beaker until the beaker and contents weigh 403.5 g plus the weight of the original soil. This equates to a 1:5 dilution of extract to 0.2 M KCl, which is a more appropriate salt load for subsequent instrumental analysis by ICPAES.
Stir the solutions to homogenise and centrifuge, or filter through Whatman No. 3 filter papers (or other suitably thick, medium-speed, high-retention paper) into suitable tubes, prior to the analytical finish by a suitable technique, with ICPAES preferred.
Set up and operate the ICPAES instrument as advised by the manufacturer. Appropriate wavelengths for Ca and Mg are 430.25 nm and 285.21 nm, respectively. The most likely wavelength for S is 182.04 nm, but other wavelengths are possible (see Method 10B3 for examples and for more details). Calibrate the instrument using a range of Combined Working Standard Solutions, guided by examples in Table 20.4. A reagent blank should also be measured and adjustments made as necessary.
Table 20.4. Volumes of Ca, Mg and S Primary and Secondary Standards required for Working Standards, plus solution and soil concentrations.

Report soluble plus exchangeable sulfur (SKCl), calcium (CaKCl) and magnesium (MgKCl) (%) on an oven-dry (85°C) basis.