The improved Suspension Peroxide Oxidation Combined Acidity and Sulfur (SPOCAS) method (McElnea et al. 2002a) supersedes the Peroxide Oxidation Combined Acidity and Sulfur (POCAS) and related methods (e.g. Latham et al. 2000). The SPOCAS method gives better recovery of S than the POCAS method and less potential for jarosite formation (McElnea et al. 2002a; McElnea et al. 2002b). Moreover, SPOCAS overcomes possible S and acid-loss problems identified by Ward et al. (2002a) and Ward et al. (2002b).
The SPOCAS acid trail involves direct determination of acidity by titration of the 1 M KCl soil suspension (1:40 ratio), whereas the S trail indirectly predicts acidity by using a combination of S determinations and stoichiometric relationships. Two measures of acidity are carried out, specifically TAA (titratable actual acidity) and TPA (titratable potential acidity). Titratable sulfidic acidity (TSA) is the difference between TPA and TAA. All three measures of acidity are conveniently expressed as mol H+/tonne. To convert from mol H+/tonne to %S, divide by 623.7 (Ahern et al. 2004b), when it is assumed all acidity is from pyrite.
The pHKCl-ASS is measured after 4 h extraction with 1 M KCl (followed by overnight standing). TAA is performed on a 1:40 soil/suspension by titration to pH 6.5 (McElnea and Ahern 2004a).
Standard ≈0.05 M Sodium Hydroxide
Dissolve 2.05 g of fresh, high-grade sodium hydroxide (NaOH) in deionised water known not to include carbonates. When the covered solution has cooled to room temperature, make to 1.0 L in a volumetric flask. Standardise against a known weight of potassium hydrogen phthalate (KHC8H4O4), previously dried for 2 h at 120°C and subsequently cooled before use in a desiccator. Note 1 and Method 4D1 provide more details on the use of KHC8H4O4 (molar = normal) as the primary reference standard. For a 5.0 mL volume of exactly 0.05 M NaOH, the equivalent weight of pre-dried KHC8H4O4 is 0.0511 g. Subsequent calculations should use the actual concentration of the NaOH solution.
Standard ≈0.25 M Sodium Hydroxide
Dissolve 10.10 g of fresh, high-grade NaOH in deionised water known not to include carbonates. When the solution has cooled to room temperature, make to 1.0 L in a volumetric flask. Standardise against a known weight of pre-dried potassium hydrogen phthalate (KHC8H4O4), as outlined for ≈0.05 M NaOH, except that for a 5.0 mL volume of exactly 0.25 M NaOH, the required weight of pre-dried KHC8H4O4 is 0.2553 g. Subsequent calculations should use the actual concentration of the NaOH solution.
1.0 M Potassium Chloride
Refer to Method 4C1. Alternatively, dissolve 74.55 g potassium chloride (KCl) and make to 1.0 L with deionised water.
pH Buffers
Obtain commercially or prepare as described in Method 4A1. These should cover the range from ≈pH 4.0–9.2.
Weigh accurately 2.00 g of dry soil (prepared as described earlier in this chapter) into a suitable extraction container and make a 1:40 suspension with 80 mL of aqueous 1 M KCl: include a blank in each batch. Stopper the container and extract soil mechanically (end-over-end shaker preferred) for four continuous hours. Allow the container and contents to stand overnight (for at least 12 h but no more than 16 h). Next day, resuspend contents by briefly shaking container (≈5 min) before quantitatively transferring contents to a separate titration vessel (if not titrating in the same extraction container) using a minimum volume of deionised water. The time between re-suspension and titration should be minimised to limit possible oxidation.
While stirring, measure and record the pH of the suspension (pHKCl-ASS) using a pH meter calibrated with appropriate buffers. Perform a titration to pH 6.5 with standardised NaOH solution using an appropriately calibrated pH meter or auto-titrator (see Note 2). Use the options described, depending on the measured pHKCl-ASS.
(1) If pHKCl-ASS is <4.0, titrate the suspension with stirring to pH 6.5 using the standardised 0.25 M NaOH and record titre volume (mL).
(2) If pHKCl-ASS is ≥4.0 but <6.5, titrate the suspension with stirring to pH 6.5 using standardised 0.05 M NaOH and record titre volume (mL).
(3) If pHKCl-ASS is ≥6.5, no titration is required and TAA has zero value.
Retain the titrated suspension if KCl-extractable sulfur (SKCl), calcium (CaKCl) and magnesium (MgKCl) are to be determined subsequently (see Method 20D1).
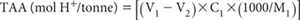
where:
V2 = Blank
C1 = Concentration of NaOH (mol/L)
V1 = Volume of NaOH titrant (mL)
M1 = Weight of sample (g)
1000 = Conversion to tonnes
Report TAA (mol H+/tonne) on an oven-dry (85°C) basis.
1. NaOH solutions should be prepared fresh daily or stored in an apparatus capable of excluding CO2. Titrations should be carried out using an auto-titrator, or manually using an A-grade 10 mL burette graduated at 0.02 mL intervals. Titrate to pH 6.5.
2. If using an auto-titrator, the volume of titrant added in each increment should decrease as the endpoint is approached. If titrating manually, titrate slowly to endpoint (pH 6.5) and wait for 20 sec. If pH drops by more than 0.1 units, back-titrate to pH 6.5.