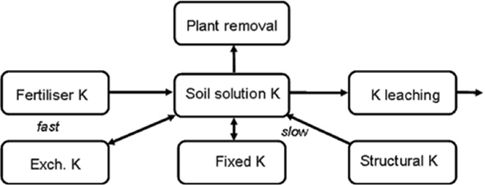
It is usual for plants to obtain their K+ from the soil solution, which in turn is replenished from both exchangeable and non-exchangeable sources within the soil profile (During and Campkin 1980; Metson 1980; Gourley 1999; Moody and Bell 2006). Potassium additions from fertilisers and manures and external losses by leaching and runoff also affect the soil–plant cycle for K+, as diagrammatically shown in Figure 18.2.
Much like Method 18C1 based on boiling 1.0 M HNO3, this tetraphenyl boron method targets ‘fixed’ soil K+ reserves, which can be an important source of plant-available K+, particularly when soil solution and exchangeable K+ reserves have been depleted by recent plant growth (e.g. Schulte and Corey 1965; Spencer and Govaars 1982; Jackson 1985; Carey and Metherell 2003a,b; Moody and Bell 2006).
Usually, K+ is quite soluble in aqueous solutions, once released from exchange and other components of the soil matrix. Likewise, sodium tetraphenylborate (NaTPB) is quite soluble in aqueous solution. When K+ is introduced into solutions of NaTPB, potassium tetraphenylborate (TBK) forms then precipitates, due to its very low water solubility (1.8 × 10-4 M). It follows that when NaTPB is used as a soil extractant, there is continual precipitation of K+, which helps maintain a low soil solution K+ concentration that subsequently facilitates release of fixed or reserve K+ (e.g. Carey et al. 2006). Information on the kinetics of soil K+ release by NaTPB is available (e.g. Dhillon and Dhillon 1992; Cox and Joern 1997). Potassium tetraphenylborate has the formula C24H20BK and the structure shown in Figure 18.3.
Earlier soil test methods for K+ based on NaTPB took advantage of the very low aqueous solubility of TBK and its much higher solubility in acetone and other organic solvents (e.g. Jackson 1985). The Jackson test, developed in New Zealand for use on air-dry soils, was shown to be a good predictor of the short-to medium-term availability of soil K+ for ryegrass. The inclusion of 1:1 acetone/water to re-dissolve precipitated TBK, prior to instrumental analysis, meant it was best used as a supplementary test with small batch sizes.
Figure 18.2. Key components of the soil–plant cycle for K (modified from Gourley 1999).
Figure 18.3. The chemical structure of potassium tetraphenylborate.
Later modifications by researchers such as Cox et al. (1996), Cox and Joern (1997) and Carey and Metherell (2003a,b) improved operational convenience by employing Cu2+ rather than either acetone or mercuric chloride to destroy the TBK precipitate prior to instrumental analysis for K+. The method described, evaluated by Carey et al. (2006), uses this option, with a 4 h ‘incubation time’ for routine use [TPK4h]. This choice was made mainly because the concentrations of K+ released were similar to those obtained by the Jackson test. Other ‘incubation times’ include 15 min and 60 min (Carey et al. 2000; Moody and Bell 2006) through to at least 168 h (Winzeler et al. 2008). Carey and Metherell (2003a) reported that across 24 New Zealand soils, ≈80% of soil K+ was released in the presence of excess NaTPB within 48 h, with significant declines after 168 h through to 672 h.
This TPK4h test involves use of 1.0 g of air-dry soil and 3.0 mL of Reagent A (a combination of 0.1 M NaTPB/1.7 M NaCl–0.01 M Na-EDTA), ‘incubated’ for 4.0 h at 20–25°C in a 60 mL boiling tube/flask calibrated at 50 mL. The K-extraction reaction is then halted with 11.5 mL of Reagent B (a combination of 1.0 M CuCl2 and 0.5 M NH4Cl Reagents in the proportion 1.5 mL to 10 mL, respectively), followed by boiling (or autoclaving at 105°C) for 30 min. Samples are then diluted to 50 mL, after adding 0.5 mL 50% HCl (v/v) (to prevent precipitation of Cu salts) then mixed thoroughly, left to stand overnight to settle particulates, and decanted or filtered and further diluted 1:5 prior to instrumental analysis for K+, using AAS, FES or ICPAES. New Zealand derived interpretations for the test are provided in Table 18.5.
0.2 M sodium tetraphenylborate (NaTPB)
Weigh 34.22 g sodium tetraphenylborate [NaB(C6H5)4; also termed sodium tetraphenyl-boron] and dissolve in deionised water, then dilute to 500 mL in a volumetric flask (see Note 1).
Table 18.5. General ratings (≈air-dry soil values) for the interpretation of soil chemical results and for use in reports of soil fertility status for reserve soil K+ (TBK4h).
TPK4h Rating |
mg K+/kg |
cmolcK+/kg |
Very low |
<120 |
<0.3 |
Low |
120–275 |
0.3–0.7 |
Medium |
>275–510 |
>0.7–1.3 |
High |
>510 |
>1.3 |
3.4 M Sodium Chloride and 0.02 M EDTA
Weigh and dissolve 99.353 g sodium chloride (NaCl) in about 350 mL deionised water. Separately dissolve 3.7225 g di-sodium EDTA {[CH2.N(CH2.COOH).CH2.COONa]2.2H2O} in a separate small quantity of deionised water, then combine the two solutions quantitatively, mix well, then make to 500 mL with deionised water.
0.5 M Ammonium Chloride
Weigh 26.75 g ammonium chloride (NH4Cl) and dissolve in deionised water then make volume to 1.0 L.
1.0 M Cupric Chloride
Weight 170.483 g cupric chloride (CuCl2.2H2O), dissolve in deionised water, then make volume to 1.0 L.
50% Hydrochloric Acid v/v
In a fume cabinet, carefully and slowly add 50 mL of 10 M HCl to 50 mL deionised water and make to 100 mL: cool to room temperature.
TBK Reagent A
Just prior to use, combine and mix well equal amounts of 3.4 M NaCl and 0.02 M EDTA Reagent and 0.2 M NaTPB Reagent. This solution corresponds to 0.1 M NaTPB/1.7 M NaCl–0.01 M Na-EDTA.
TBK Reagent B
Combine sufficient 1.0 M CuCl2 Reagent and 0.5 M NH4Cl Reagent in the proportion 1.5 mL to 10 mL, respectively.
Potassium Primary Standard–TBK
1 L contains 1000 mg of K.
Dissolve 1.9068 g potassium chloride (KCl; previously dried at 110°C for 2 h) in deionised water and make to 1.0 L in a volumetric flask. Store in a clean, well-sealed plastic bottle.
Potassium Working Standards–TBK
To generate a matrix-matched solution for each of the K Working Standards–TBK (when each working standard is diluted to 500 mL with deionised water), initially add ≈100 mL deionised water to each volumetric flask, followed by 30 mL of TBK Reagent A, 115 mL of TBK Reagent B and 5 mL of 50% HCl v/v, then mix. (These reagents should be from the same batches used for unknown soils.) Next accurately dispense 0, 0.5, 1.0, 2.5 and 5.0 mL of K Primary Standard–TBK into the prepared 500 mL volumetric flasks, mix well and make each to 500 mL with deionised water. Potassium concentrations in Working Standards–TBK are 0, 1.0, 2.0, 5.0 and 10.0 mg K+/L. For a 1:50 soil/extract ratio (and excluding any subsequent dilutions), these Working Standards–TBK correspond to 0, 50, 100, 250 and 500 mg K+/kg of air-dry soil. The Working Standards have a shelf life of 6 months when stored in sealed, polypropylene reagent bottles. Discard if visible mould develops.
Weigh 1.0 g air-dry soil (<2 mm) into labelled 60 mL borosilicate boiling tubes/flasks, each with an accurate graduation mark at 50 mL. Also include quality control samples and at least two reagent blanks in each batch of 50 samples. Add 3 mL of Reagent A and ‘incubate’ the batch of samples at between 20–25°C for exactly 4 h, preferably with end-over-end mechanical shaking.
Remove from the shaker and add 11.5 mL of Reagent B to each sample, swirl to mix, cover the digest tubes (located in a rack) with clean Al foil, then place the batch of covered samples (in digest tubes) onto a heat-regulated hot plate and boil gently for 30 min (or autoclave at 100–105°C for 30 min; see Note 2).
After boiling or autoclaving, cool the tubes and contents, then add, without further delay, 0.5 mL of 50% HCl v/v to each tube. Next stir all unknown samples using a vortex mixer (or equivalent) to ensure all of the precipitate of TBK in each digest tubes is thoroughly dissolved. When so, make each to 50 mL with deionised water, stopper and shake.
Allow the samples to stand to ensure a clear supernatant is achieved (this is conveniently achieved by overnight standing: see Note 3).
Carefully and accurately remove 2 mL of extract into clean, small mixing tubes containing 8.0 mL of deionised water and mix well. Do likewise for each K Working Standard–TBK.
Present diluted K Working Standards–TBK and diluted soil extracts for instrumental analysis of K+. Analyse by FES, AAS, or ICPAES, using procedures similar to those described in Method 15A1/18A1 or as recommended by the instrument manufacturer: see Note 4. For ICPAES, use one of two optional spectral lines (primary wavelength = 769.897 nm; secondary wavelength = 766.491 nm), with 0.5% (w/w) lithium nitrate as the ion suppressant.
TBK4th (mg K+/kg air dry) = (mg K/kg in sample) − (mg K/kg in blank)
Alternatively:
TBK4th (cmolc K+/kg air dry) = {[(mg K/kg in sample) − (mgK/kginblank]/391}
In both of the above, take into account any subsequent dilutions from the initial soil weight and initial extract volume.
Report TBK4h as either mg K+/kg air-dry soil, or as cmolcK+/kg air-dry soil. Should the ‘incubation time’ differ from 4.0 h, then report the actual ‘incubation time’, together with the numeric result. For example, for an ‘incubation time’ of 15 min, report as TBK15min, followed by the numeric value.
1. The volume is offered only for guidance. It is suggested that operators only prepare the minimum amount of reagent needed at the time, as NaTPB is quite costly. Also, it is essential that operators handle the reagent with care (use protective gloves and work in a fume cupboard) due to the toxicity of the reagent. It is harmful if swallowed, inhaled or if it makes contact with the skin or eyes.
2. During local method development, attach one or two Cole ParmerTM Irreversible Indicating Temperature Labels designed to operate at 8 points from 71–110°C to each rack/batch of samples to ensure the correct boiling/autoclaving temperature is consistently achieved. When there is reasonable confidence in temperature settings and times, continue to occasionally (e.g. 3 monthly) monitor temperatures using Cole ParmerTM Irreversible Indicating Temperature Labels or similar.
3. It is imperative that the 50 mL sample solution is allowed to stand for sufficient time to achieve particulate-free supernatants. This is to ensure that the final extract solution presented to the measurement instrument does not damage or block the nebuliser.
4. A wide range of cations and anions do not interfere in K+ extraction and analysis involving NaTPB. A few, such as Hg2+, Hg+, Ag+, Cu+ and occasional N-containing organic compounds, however, may interfere if present. Interference from sodium perchlorate is unlikely. Lithium nitrate (LiNO3) at 0.5% w/w is an effective ion suppressant for axial ICP, although lower concentrations of Li+ may suffice when using AAS (P Lorentz, pers. comm.).