The bicarbonate-extractable P test of Colwell (1963), based on 0.5 M sodium bicarbonate (NaHCO3) at pH 8.5, was in commercial use by the mid-1960s (Fogliati 1967) as an aid to the responsible marketing of fertilisers in New South Wales. The opportunity to obtain an estimate of extractable K+ in the same soil extract was quickly recognised. For example, Colwell and Esdaile (1968) reported that for a wide range of soils, the K+ extracted was highly correlated (r = 0.96) with exchangeable K+ based on an ammonium chloride (NH4Cl) extraction at pH 7.0. Nowadays, this test, sometimes referred to as Colwell–K, is the ‘standard’ diagnostic K test used in Western Australia (Bolland et al. (2002).
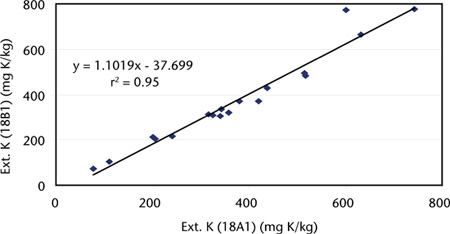
Figure 18.1, based on sample median data from inter-laboratory proficiency programs of ASPAC reported from 1993–1997 (e.g. Peverill and Maheswaran 1993; Peverill and Johnstone 1997), shows a strong, linear relationship between soil K+ extracted by this method and by Method 18B1 (4 h extraction with 0.05 M HCl).
Figure 18.1. Comparisons of median values (air-dry) of soil-extractable K+ by Methods 18A1 and 18B1, obtained from inter-laboratory proficiency programs of ASPAC from 1993–1997. Grand median values were 350 and 329 mg K/kg for methods 18A1 and 18B1, respectively.
Table 18.1. Summary details of method codes, method titles, technologies and notes on miscellaneous tests described in this chapter.
Code |
Technology |
Test method |
Notes |
18A1 |
Empirical test using Colwell P extract (0.5 M NaHCO3 at pH 8.5, 1:100 soil/solution ratio; 16 h), with instrumental analysis of K+. |
Bicarbonate-extractable K+. |
Useful and economical soil K test, where the laboratory is already committed to perform Colwell P extractions. |
18B1 |
Empirical test using 0.05 M HCl extraction (4 h at 1:40 soil/solution ratio) with instrumental analysis of K+. |
Hydrochloric acid-extractable K+. |
Useful and economical soil K+ test, particularly for use on neutral and acidic soils. Unsuitable for use on highly alkaline soils. |
18C1 |
Empirical test for non-exchangeable K+ (boiling 1.0 M HNO3 at initial 1:40 soil/solution ratio; 30 min) with instrumental analysis of K+. |
Boiling 1.0 M nitric acid-extractable K+. |
Provides a measure of non-exchangeable K+ relevant to sugar cane, particularly when grown on neutral and acidic soils. |
18D1 |
Empirical tests for phyto-available soil Cd based on extractions with CaCl2 and subsequent instrumental analysis of Cd. |
0.1 M CaCl2- extractable Cd (2 h extraction at 1:2.5 soil/extract ratio). |
Useful test to predict phyto-available soil Cd, particularly by laboratories without access to ICP-MS. Slightly higher concentrations than from Method 18E1 makes it analytically attractive. |
18E1 |
|
0.01 M CaCl2- extractable Cd (4 h extraction at 1:5 soil/extract ratio). |
Useful test to predict phyto-available soil Cd by laboratories with ICP-MS and when soils are highly weathered (low ionic strength). |
18F1 |
Multi-element empirical test on air-dry soil, with analysis by ICPAES |
Mehlich 3 extractable elements (P, Ca, Mg, Na, K, Fe, Cu, Mn, Zn, B, S, Al). |
Widely used internationally for rapid, cheap, comprehensive soil fertility assessments (cations, macro- and micro-nutrients). |
18F2 |
Part of multi-element empirical test (colorimetric for extractable P). |
Mehlich 3 extractable P – colour finish. |
Optional analytical finish for Mehlich 3 extractable P, for labs without access to ICPAES or when soil P calibrations were based on a colorimetric finish. |
18G1 |
Empirical test for reserve soil K+ by Cu-modified sodium tetraphenylboron extraction, with instrumental analysis of K. |
Reserve soil K+ by Cu-modified sodium tetraphenylboron extraction [TBK4h]. |
An emerging soil test for ‘reserve’ soil K, influenced by ‘incubation time’. This test is based on 4 h ‘incubation’ but there is provision for other times. |
Table 18.2. General ratings from Rayment (2003) for the interpretation of both bicarbonate-extractable K+ and acid-extractable K+ (cmolcK/kg) in air-dry surface soils from northern Australia.
Rating |
Value/range |
Very low |
<0.1 |
Low |
0.1–0.2 |
Medium |
>0.2–0.5 |
High |
>0.5–1.0 |
Very high |
>1.0 |
Use of Method 18A1 is justified when comprehensive analysis to assess the ion exchange properties of soils is unavailable or unwarranted and when the test results are known to relate to crop response to applications of potash fertiliser. Examples of soil K fertility ratings are provided in Table 18.2.
Method 18A1 involves the measurement of K+ in clarified extracts of 0.5 M NaHCO3 at pH 8.5, following 16 h extraction, with subsequent analysis by flame photometry, AAS or ICPAES. Alternatively, K+ may be determined by flame FES incorporated into a continuous segmented flow system. Analytically, the wide soil/solution ratio of 1:100 can be a disadvantage when measuring K+ concentrations in extracts from soils low in soluble plus exchangeable K+.
Extracting Solution – 0.5 M Sodium Bicarbonate at pH 8.5
As for Method 9B1.
Li Reagent (for auto flame-photometer)
Dissolve 4.0 g lithium sulfate (Li2SO4.H2O; reagent grade) in deionised water and make volume to 1 L. Add 4 drops of Brij 35 Wetting Agent (see Method 5A2a for preparation details). Add 10 mL/L ammonium hydroxide (NH4OH; 28–30%; reagent grade) for pH control of the reagent to the auto-flame photometer. Specifically, check the pH of the recipient stream on each day of use (universial pH indicator paper is sufficiently accurate for this purpose) and adjust the pH of the Li Reagent so as the pH of the recipient stream is near neutral.
Potassium Primary Standard
1 L contains 500 mg of K+.
Dissolve 0.9534 g potassium chloride (KCl; previously dried at 110°C for 2 h) in deionised water and make to 1.0 L in a volumetric flask. Store in a clean, well-sealed plastic bottle.
Potassium Secondary Standard
1 L contains 100 mg of K+.
Dilute 100 mL K Primary Standard to 500 mL with 0.5 M NaHCO3 at pH 8.5 Extracting Solution in a volumetric flask. Freshly prepare when K Working Standards are required.
Potassium Working Standards
Dispense 0, 1.0, 2.0, 5.0, 10.0, 20.0, 30.0, 40.0 and 50.0 mL K Secondary Standard into separate 500 mL volumetric flasks and make to volume with 0.5 M NaHCO3 at pH 8.5 Extracting Solution. These Working Standards cover the range 0–10 mg K+/L and for a 1:100 soil/solution ratio are equivalent to soil concentrations of 0, 20, 40, 100, … 1000 mg K+/kg. Store in clean, sealed plastic bottles.
Weigh and extract air-dry soil (<2 mm) with 0.5 M NaHCO3 at pH 8.5 as described in Method 9B1, ensuring that apparatus and filter paper (if applicable) are free of K+ contamination/residues.
Analyse for K+ in the clarified aliquots and K Working Standards after acidic dilution (see Note 1) by FES, AAS, or ICPAES, using instrumental procedures similar to those described in Method 15A1.
If an automated flame photometer is available, a neutralisation step with 0.75 M H2SO4 – as shown in Figure 9.4 – is warranted, prior to addition of Li Reagent as an internal standard. A typical continuous segmented-flow manifold for K+ is shown in Figure 15.2.
Report bicarbonate-extractable K+ (mg K+/kg) on an air-dry basis. To convert to cmolcK/kg, divide mg K+/kg by 391.
1. Undiluted 0.5 M NaHCO3 carries a high ‘salt’ burden, often sufficient to extinguish an ICPAES plasma and to cause a build-up of ‘salt’ deposits on the ICPAES torch and on burners during FES and AAS analysis, particularly when large batch-sizes of samples are involved. These effects can be minimised by at least 1+1 dilution with dilute H2SO4 of the clarified extracts and working standards prior to instrumental analysis, so the final diluted extract/working standards are approximately pH neutral. This procedure should be undertaken in a manner that permits the dissipation of CO2 released as a consequence of acidic dilution.
Figure 9.4. A continuous, segmented flow sheet (AutoAnalyzer I/II technology) for Colwell-P
Figure 15.2. Continuous segmented flow manifold for Na+ and K+ in 1 M NH4Cl soil extracts.