In addition to sulfate, organic S compounds can contribute to the S nutrition of crops and pastures. Sulfate esters that are readily mineralised are a case in point. Moreover, potassium chloride (KCl) at 40°C for 3 h has emerged as an alternative to monocalcium phosphate (MCP) as a useful soil chemical test for sulfate-S. This is because 0.25 M KCl at 40°C will extract sulfate-S from soil solution, from easily exchanged and adsorbed surfaces, and from easily mineralised ester sulfates (Blair et al. 1991; Lefroy et al. 1993). What is more, Lefroy et al. (1993) reported higher correlation with % maximum yield of pastures from 47 sites, most from northern New South Wales. Chinoim et al. (2003) reported the test generally performed well when used to assess the S supply to three crops on two soils on the Northern Tablelands of New South Wales.
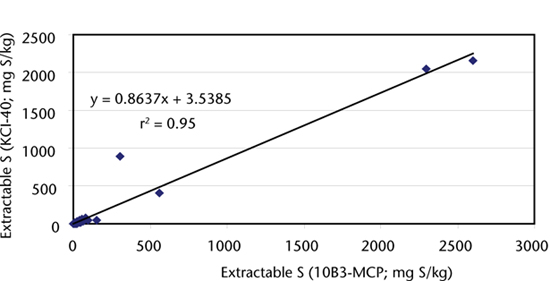
Lefroy et al. (1993) reported a (KCl-40) value of 6.4 mg S/kg at 90% maximum yield, with the corresponding MCP-S value of 8.2 mg S/kg. This is a 1:1.28 ratio, whereas for 72 well prepared samples from Australia and New Zealand included in a decade of inter-laboratory proficiency testing, the ratio between the two tests – 10D1-(KCl-40) vs 10B3 (MCP) – was 1:1.5. With one exception, there was a strong linear relationship between the two tests (Figure 10.4) across a wide concentration range. The measurement performance of Method 10D1 in ASPAC inter-laboratory proficiency programs for over a decade (e.g. Peverill and Johnstone 1997; Lyons et al. 2008) was slightly superior to that of Method 10B: see Figures 10.1 and 10.5. The method is applicable to all soils and S in the KCl-40 extracts is determined by ICPAES.
Figure 10.4. Continuous linear relationship between extractable S by methods 10B3 and 10D1.
0.25 M Potassium Chloride Extracting Solution
Dissolve 18.64 g potassium chloride (KC1) in deionised water and make to 1.0 L. Use this solution for soil extractions, as the reagent blank and in working standard solutions.
Sulfate Primary Standard
1 mL contains 1 mg of S.
As for Method 10B1.
Sulfate Secondary Standard Solution
1 mL contains 200 μg of S.
Take 200 mL of SO4 Primary Standard from Method 10B1 and dilute to 1.0 L with 0.25 M KCl Extracting Solution. Prepare on the day of use.
Sulfate Working Standards-KCl-40
Prepare by adding 0, 1.25, 2.5, 5.0, 10.0, … 75.0 and 100.0 mL of freshly prepared SO4 Secondary Standard to separate 500 mL volumetric flasks. When diluted to 500 mL with 0.25 M KCl Extracting Solution these working standards contain 0, 0.5, 1.0, 2.0, 4.0, … 30.0 and 40.0 mg S/L. Equivalent soil strengths for a 1:6.67 soil/solution ratio are 0, 3.3, 6.7, 13.3, 26.7 …200 and 267 mg S/kg, respectively.
Weigh 4.50 g air-dry soil (<2 mm) into a 50 mL polypropylene (ElKay) vial with screw cap. Add 30 mL of 0.25 M KCl Extracting Solution, preheated to ≈40°C, seal with the screw cap, and shake vial by hand to ensure the soil is thoroughly wet and dispersed. Transfer to a pre-heated laboratory oven/incubator calibrated to 40±0.5°C and equilibrate continuously at this temperature for 3 h. Remove from oven and again shake each vial briefly to redisperse the soil.
Figure 10.5. KCl-40-extractable sulfate-S concentrations (Method 10D1; ICPAES) vs % robust CVs (1997–2007) derived from ASPAC soil inter-laboratory proficiency programs. Two samples with concentrations >2000 mg S/kg are not presented but are included in the continuous trend line.
Centrifuge at about 3000 rpm for 20 min or filter extracts through Whatman No. 40 paper (discarding the first portion) into a 120 mL plastic (polycarbonate) vial. If 11 cm paper is used, no funnel will be required as the paper will be self supporting. No particulate matter should pass through the filter.
Allow the KCl-40 extracts to re-equilibrate to laboratory room temperature (≈25°C), then determine S concentrations in these extracts, in a selection of Sulfate Working Standards-KCl-40, and in blanks and control samples using ICPAES.
Follow manufacturer’s instructions for instrument settings and for the preferred wavelength. The most likely wavelength is 182.037 nm, but other wavelengths are possible, such as 180.731, 182.037, and 182.625 nm. Avoid using the S emission doublet at 182.619/182.635 nm. At 182.037 nm, virtually no interference from Ca2+ is expected, noting that Ca2+ concentrations in the KCl-40 Extracting Solution will vary from soil to soil.
Should it be necessary to interupt the analysis, the filtered extracts can be stored in a refrigerator (≈4°C) for up to 1 week, provided extracts are suitably covered to prevent evaporation. Several layers of plastic film (tested to be free of S) are usually sufficient.
Report KCl-40-extractable S (mg S/kg) on an air-dry basis.