Sulfur is first extracted with 0.01 M Ca(H2PO4)2 at pH 4.0 as described in Method 10B1. The extracted S (mostly SO42–) is determined by the automated microdistillation method of Keay et al. (1972). In this procedure, reduction and distillation steps similar to those of Johnson and Nishita (1952) have been combined with the colloidal bismuth sulfide (BiS) finish of Dean (1966) in a SFA system. The SFA system outlined herein is based on AutoAnalyzer I/II technology. It is recognised, however, that improvements in system performance are ongoing, including faster rates of analysis, lower reagent volumes, more reliability, automatic data handling, sample dilution and speedier reagent change-over. For more details on SFA, see Gordon et al. (1993) and Section 4120 of APHA (2005).
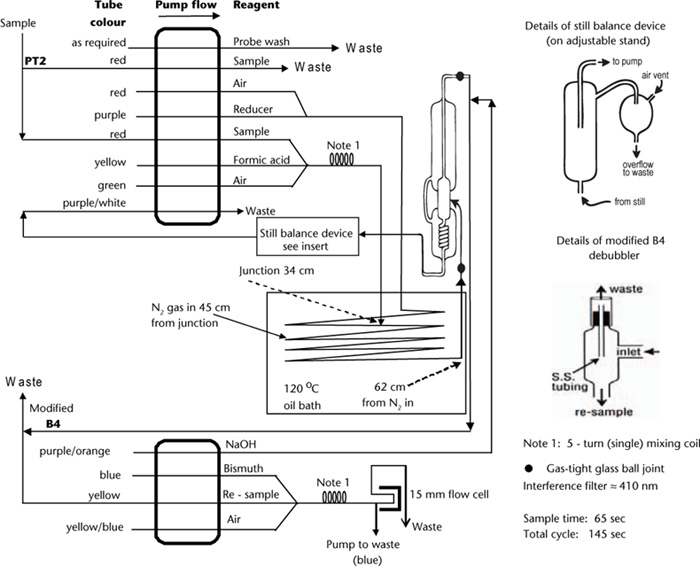
See Figure 10.3 for details of a proven continuous SFA manifold incorporating automated distillation. Modifications to the original configuration of Keay et al. (1972) are as follows:
(a) three ball-joints are used to minimise chances of breakage to the still;
(b) the NaOH inlet and NaOH/H2S absorption sections of the still are constructed from capillary glass, and
(c) an adjustable self-levelling trap is used to ensure a ‘liquid-seal’ just above the inner glass coil of the still itself. The upper level of liquid in the still should remain below the high temperature liquid inlet. Excess condensate from the trap is pumped to waste.
Extracting Solution – 0.01 M Ca(H2PO4)2 at pH 4.0
As for Method 10B1.
Reducing Agent
Mix together in a suitable 2 L refluxing flask:
500 mL 55% (w/v) hydriodic acid (HI)
250 mL 99% (v/v) formic acid (HCOOH)
125 mL 50% hypophosphorous acid (H3PO2)
Boil carefully below 117°C under reflux in a fume cupboard with a stream of N2 gas bubbling throughout the solution. Continue boiling for about 10 min after the solution has cleared. When cool, disconnect the N2 supply and transfer to a dark storage bottle. If protected from light and atmosphere, the reagent remains stable for 1–2 months.
Brij 35 Wetting Agent
As for Method 5A2.
Dissolve 40 g sodium hydroxide (NaOH) in CO2 free (boiled) deionised water and make to 1 L. Add 1 mL Brij 35 wetting agent.
Formic Acid (HCOOH)
99% formic acid.
Acetic Acid (CH3CCOOH)
Glacial (99.5%) acetic acid.
Bismuth Reagent
Heat 2 g bismuth subnitrate (BiONO3.H2O) or 3.4 g bismuth nitrate pentahydrate [Bi(NO3)3.5H2O] with 460 mL of glacial CH3COOH until it has dissolved. Filter solution through a No. 50 Whatman filter paper, cool, and add 500 mL of previously boiled and cooled deionised water containing 6 g gelatine that has been previously dissolved by warming. Dilute the mixture to 2 L.
Sulfate Standard Solutions
As for Method 10B1.
Prepare particle-free soil extracts as described for Method 10B1 and transfer to clean dry tubes for continuous SFA. Also ensure individual components of the continuous flow analyser system for the determination of S are connected as detailed on the flow sheet. Refer to Figure 10.3 and to Notes 1-5.
Operate the AutoAnalyzer in accord with manufacturer’s instructions. ‘Condition’ the manifold and the still before use and check instrument, heating bath and N2 gas settings, then run calibration standards on commencement, after at least every 30 soil extracts and on completion. If necessary, dilute over-range extracts with 0.01 M Ca(H2PO4)2 at pH 4.0 Extracting Solution. Changing the initial soil/solution ratio is not an option. Also use Extracting Solution as the wash solution during auto analysis. Use calibration curves or regression equations to obtain results.
Report phosphate-extractable S (mg S/kg) on an air-dry basis.
1. A constant flow-rate of N2 during continuous SFA is critical. A rate of 100–120 mL/min is normally sufficient. This should be set by a gas flow meter, with a liquid trap incorporated just prior to the still inlet. The liquid trap is to protect the gas flow meter from ‘suck-back’ when the system is shut down.
2. Acidflex pump tubes and Acidflex sleeving and transmission tubing are recommended for pumping waste from the still and for connecting glass tubes carrying Formic Acid and Reducing Reagent, respectively. In general, glass transmission tubing should be used whenever possible.
3. Make regular checks to ensure the still remains ‘balanced’. Changes in atmospheric pressure can affect this setting.
4. Pump tube selections for sample and reagents are optimised for a maximum SO4-S concentration of 50 mg S/kg. Deposition of BiS in mixing coils and flow-cell can occur when samples contain higher S concentrations; baseline drift is the result. If a significant number of samples are expected to exceed 50 mg S/kg, a smaller sample pump tube should be selected. An additional pump tube to supply deionised water such that the new sample volume plus water is equivalent to the original 0.8 mL/min should be added prior to the first mixing coil. Also extend the range of SO4-S Working Standards.
Figure 10.3. Calcium phosphate-extractable S autoanalysis flow sheet (AutoAnalyzer I/II technology).
5. If cell and associated transmission lines become coated with BiS, these coatings must be removed. A 1+4 HNO3/H2O solution should be pumped for 3–5 min through the BiS segment of the manifold; remove the return tube from the still (NaOH) to prevent neutralisation of the dilute HNO3 solution.
6. This automated Johnson and Nishita analytical finish can be readily adapted to measure S in clarified extracts of Method 10C1 and 10D1.