Sulfate S is extracted in the absence of activated charcoal from air-dry soil of <2 mm particle size by 0.01 M Ca(H2PO4)2 at pH 4.0 (Fox et al. 1964; Barrow 1967; Beaton et al. 1968) using a soil/solution ratio of 1:5 and an extraction time of 17 h at 25°C. This extracting solution contains sufficient phosphate ions to displace adsorbed S from all but very strongly sorbing soils (Barrow 1967).
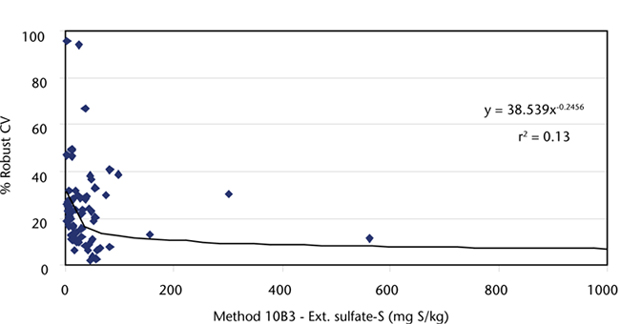
Figure 10.1. Soil-extractable sulfate-S concentrations (Method 10B3; ICPAES) vs robust % CVs (1997–2007) derived from ASPAC soil inter-laboratory proficiency programs. Two samples with concentrations >2000 mg S/kg are not shown but are included in the continuous trend line.
The extracted S is then determined in an aliquot of particle-free soil extract by the method of Johnson and Nishita (1952). Sulfate ions are reduced to hydrogen sulfide (H2S) and the evolved gas absorbed in a solution of Zn2+ and sodium acetate (CH3COONa). The sulfide anion is then allowed to react with p-aminodimethylaniline sulfate and H+ to form methylene blue (reduced form) in the presence of Fe3+, permitting the colorimetric measurement of phosphate-extractable S.
Extracting Solution – 0.01 M Ca(H2 PO4)2 at pH 4.0
To prepare 50 L of extracting solution:
(a) Calcine approximately 50 g calcium carbonate (CaCO3) by placing a sufficient quantity into a furnace at 800°C. Raise temperature to 1000°C and maintain temperature for 45 min. Cool in a desiccant-free desiccator then add 60 g calcined calcium carbonate (CaO) to 30 L deionised water, stopper, shake well, and allow the Ca(OH)2 solution formed to stand overnight.
(b) Weigh 116.82 g phosphoric acid (H3PO4) and transfer to a container holding not more than 15 L deionised water.
(c) Add ≈22 L of suspension-free Ca(OH)2 solution (a) in 2 L aliquots with constant stirring to the dilute H3PO4 solution (b). Adjust pH to 4.0 with either dilute H3PO4 or Ca(OH)2 solution. Make to volume with deionised water: see Note 1.
Reducing Agent
Mix together in a suitable 2 L refluxing flask:
600 mL 55% (w/v) hydriodic acid (HI)
150 mL sg. 1.13 hypophosphorous acid (H3PO2)
300 mL 90% (v/v) formic acid (HCOOH)
Boil carefully below 117°C under reflux in a fume cupboard with a stream of N2 gas bubbling throughout the solution. Continue boiling for about 10 min after the solution has cleared. (If boiled above 117°C, highly poisonous phosphine gas (PH3) may form.)
When cool, disconnect N2 and transfer the reducing agent to a dark storage bottle. If protected from light and atmosphere, the reagent remains stable for 1–2 months. Used reducing agent may be regenerated in a similar way up to a total of three times, provided its NO3-N content remains low.
Absorbing Solution
Dissolve 50.0 g zinc acetate dihydrate [(CH3COO)2 Zn.2H2O] and 12.5 g sodium acetate trihydrate (CH3COONa.3H2O) in deionised water and make to 1 L. Filter (Whatman No. 42 paper) before use.
Aminodimethylanaline Solution
Dissolve 1.0 g N, N-dimethyl-p-phenylenediamine sulfate [NH2C6H4N(CH3)2]2.H2SO4 in 70 mL deionised water. Add carefully 200 mL 18 M H2SO4 in small portions, cooling and mixing between additions. Cool and make to 1 L in a volumetric flask. Avoid free base formation during preparation, as this chemical is a mild vesicant (blisters or burns body tissues by contact with skin or following inhalation).
Ferric Iron Solution
To 125.0 g ferric ammonium sulfate [FeNH4(SO4)2.12H2O] add 25 mL 18 M H2SO4 and 975 mL deionised water. Invert several times to dissolve.
Gas Washing Solution
Dissolve 10.0 g sodium dihydrogen orthophosphate (NaH2PO4.2H2O) and 10.0 g pyrogallol [C6H3(OH)3] in 100 mL deionised water with the aid of a stream of N2 gas bubbling through the solution. Keep well sealed prior to use. Make fresh weekly or when solution in the gas washing column is highly discoloured.
Sulfate Primary Standard
1 mL contains 1 mg of S.
Dissolve 5.4353 g potassium sulfate (K2SO4; previously dried at 105°C for 4 h) in deionised water and make to 1 L in a volumetric flask. Add 2 drops chloroform (CHCl3) to suppress biological activity and store solution in borosilicate glass, preferably in dark.
Sulfate Secondary Standard
1 mL contains 100 μg of S.
Take 50 mL SO4-S Primary Standard and dilute to 500 mL with 0.01 M Ca(H2PO4)2 at pH 4.0 Extracting Solution. Retain after preparing working standards and use the excess to ‘condition’ the Johnson and Nishita apparatus.
Sulfate Working Standards
Prepare by adding 0, 2.5, 5.0, 10.0, 20.0, 30.0, 40.0 and 50.0 mL of freshly prepared SO4-S Secondary Standard to separate 500 mL volumetric flasks. When diluted to 500 mL with Extracting Solution these working standards contain 0, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0 and 10.0 mg S/L. Equivalent soil strengths for a 1:5 soil/solution ratio are 0, 2.5, 5.0, 10.0, 20.0, 30.0, 40.0 and 50.0 mg S/kg, respectively.
The apparatus shown in Figure 10.2 is based on Johnson and Nishita (1952). A gas delivery tube is connected to the U tube of the gas washing column by a short piece of plastic tubing. The delivery end of the gas delivery tube is tapered, allowing it to reach the bottom of a 100 mL volumetric flask. Heating is by micro-burner via a suitable gauze. All ground glass joints should be lubricated with S-free ground glass joint lubricant: see Note 2.
Install a gas washing bottle between the N2 cylinder and the apparatus inlet. Replace the Gas Washing Solution weekly or when badly discoloured.
Weigh 20.0 g of air-dry soil (<2 mm) into a 250 mL plastic bottle. Add 100 mL Extracting Solution [0.01 M Ca(H2PO4)2 at pH 4.0], stopper, and shake end-over-end for 17 h at 25°C. Centrifuge at about 3000 rpm for 20 min or filter extracts through Whatman No. 42 paper, discarding the first portion.
Clean the Johnson and Nishita apparatus thoroughly with deionised water (detergent washing recommended following periods of storage) and drain. Grease all Quickfit male joints of the apparatus with a minimum of S-free lubricant. Add 10 mL Gas Washing Solution to each column and assemble the apparatus as shown in Figure 10.2 using Quickfit springs where necessary. Turn on condenser water, gas burners and adjust N2 flow rate to ≈150 mL/min (3 to 4 bubbles/sec). Check periodically.
Figure 10.2. Modified Johnson and Nishita apparatus for calcium phosphate-extractable S.
To ensure H2S saturation of the liquid system, condition the apparatus each day by adding initially 0.5 mL SO4-S Secondary Standard to the round-bottom reduction flask. Next add 2 glass beads and 4 mL Reducing Agent and quickly attach the flask to the condenser and heat to boiling within 60 sec. Reduce the heat and allow to reflux for ≥30 min. (A receiving vessel is not required at this stage).
Prepare a series of 100 mL volumetric flasks containing 10 mL of Absorbing Solution and about 70 mL deionised water. When the apparatus has been conditioned, raise one of these volumetric flasks under the gas delivery tube of each apparatus until the outlet almost touches the bottom of the volumetric flask.
Pipette a suitable aliquot of soil extract (or working standard), commonly 5.0 mL, to a reducing flask. Ensure the flask top is lightly greased, add glass beads and, immediately prior to connecting to the apparatus for refluxing, add 4.0 mL Reducing Agent. Quickly connect flask to the condenser with Quickfit spring. Heat to boiling within 60 sec then reduce heat and allow to reflux for 60 min at a low boil. Check the N2 gas flow rate periodically during this period. When S concentrations are low, take a larger aliquot and evaporate to dryness before adding the reducing agent: see Note 3.
Detach the glass delivery tube from the plastic sleeve so that the delivery tube drops gently into the 100 mL flask. Pipette 10 mL aminodimethylanaline solution into the 100 mL flask, stopper and shake, then add 2 mL Ferric Iron Solution, restopper and shake.
Remove the glass delivery tube from the 100 mL flask with a pair of tweezers and rinse clean with deionised water. Make volume to 100 mL with deionised water and shake well. Allow at least 10 min for full colour development: colour remains stable for up to 24 h.
Detach the reducing flask, allow it to cool, then pour the used reducing agent via a grooved funnel (to catch glass beads) into a suitable dark storage bottle for later regeneration.
When fully drained, reattach the gas delivery tube to the apparatus and repeat the operations on equal volumes of the extracting solution, soil extracts, and working standards. When operations for the day are complete, shut-down the apparatus: see Notes 4 and 5.
Read absorbance of both standards and soil extract solutions in the range 660–670 nm on a suitable spectrophotometer.
Plot absorbance of working standards against S concentrations (or use a regression equation) to obtain S concentration of soil extracts. For a 1:5 soil/solution ratio and equal aliquots of soil extracts and standard solutions, S concentrations can be obtained directly. Adjust for any reagent blank.
Report phosphate-extractable S (mg S/kg) on an air-dry basis.
1. The theoretical quantity of saturated Ca(OH)2 solution required for 50 L of 0.01 M Ca(H2PO4)2 extracting solution is 24 L, but this varies with temperature. The solubility of Ca(OH)2 increases with decreasing solution temperature.
2. To remove S from ground-glass joint lubricant, mix ≈5 g grease with 5 mL of both HI and H3PO2 in a 50 mL beaker. Fill a round bottom Kjeldahl flask with cold water and sit it on top of the beaker to act as a condenser. Boil the mixture with frequent stirring for ≈45 min. Pour off the acid and wash the lubricant thoroughly with deionised water.
3. Where large aliquots of soil extracts are necessary (due to low S concentration) the extract solution should be evaporated to dryness prior to the addition of Reducing Agent. Evaporation may be accelerated by drying in an oven at 130°C or by blowing a stream of air, washed in 4 M KOH (224.4 g/L) into the upper part of the flask.
4. Leaving the reducing flask connected to the condenser following the last reflux on the previous day stops impurities entering the apparatus and maintains an inert atmosphere (N2), obviating the need to thoroughly clean the apparatus prior to use.
5. When turning off the apparatus, concurrently turn off the gas at the supply outlet and remove the N2 delivery tube. Next turn off the N2 gas supply. Finally shut off the water supply to the condenser.
6. This manual Johnson and Nishita analytical finish can be readily adapted to measure S in clarified extracts of Method 10C1 and 10D1.