Segmented, continuous flow analysis technology is broadly described elsewhere (e.g. Gordon et al. 1993; Section 4120 of APHA 2005). The SFA herein outlined is an example based on AutoAnalyzer I/II technology. It is recognised, however, that improvements in system performance are ongoing, including faster rates of analysis, lower reagent volumes, more reliability, automatic data handling, sample dilution and speedier reagent change-over. Reductions in dispersion have centred on smaller internal diameters of reaction tubing, down from 2.4 mm in the Technicon AutoAnalyzer I, to 2 mm in the AutoAnalyzer II, and to 1 mm in more modern instruments (Jodo et al. 1992). The latest systems incorporate a better understanding of the way in which dispersion, tubing diameter and flow rate are interrelated (Gordon et al. 1993).
To accommodate the automated colorimetric finish, the Kjeldahl digest described in Method 7A1 is diluted to a known volume. The NH4-N and any free amino acids in diluted digest solutions are measured colorimetrically at 660 nm by the nitroprusside/dichloro-S-triazine modification (Searle 1975; Blakemore et al. 1987) of the Berthelot indophenol reaction reviewed by Searle (1984). Free amino groups that can react are most likely to be present in soils that have been fumigated or dried at elevated temperatures (Burton et al. 1989).
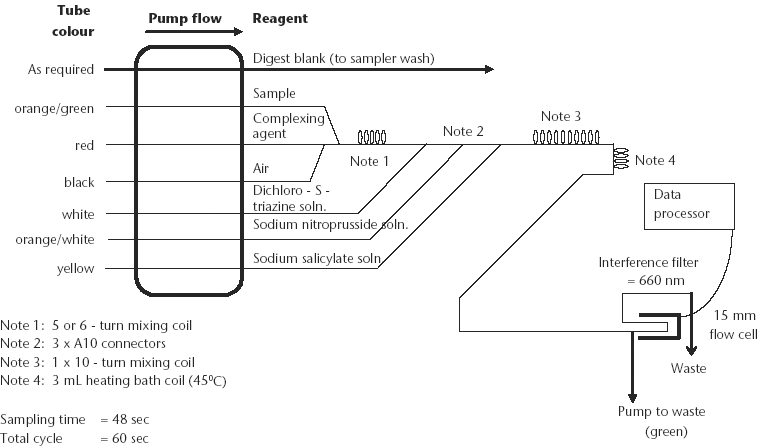
The continuous flow instrumentation uses a peristaltic pump to draw sample extracts from the sampler into a manifold designed to mix the samples with reagents that are being pumped at predetermined flow rates, and on eventually to the colorimeter detector. It is acknowledged that the analyst must be guided by the operational instructions and directions given by the manufacturer. This could require changes to the specified reagents and to the flow diagram provided for this method.
Digest Acid
As for Method 7A1.
Catalyst Mixture
As for Method 7A1.
Sodium Nitroprusside Reagent
Dissolve 1.2 g sodium nitroprusside dihydrate {Na2[Fe(CN)5NO].2H2O} in deionised water and dilute to 500 mL.
Dichloro-S-triazine Solution
Dissolve 10.0 g sodium hydroxide (NaOH) in 400 mL deionised water, add 0.25 g of 1-sodium-3,5-dichloro-S-triazine-2,4,6-trione (C3HCl2N2O3.Na) and make to 500 mL. The active chemical in this reagent is also called sodium dichloroisocyanurate. Commercial grade reagent is satisfactory.
Sodium Salicylate
Dissolve 15.0 g sodium salicylate [C6H4(OH)COONa] in deionised water and make to 1 L.
Citrate–Tartrate Complexing Agent
Dissolve 25.0 g NaOH in 800 mL deionised water. Add 6.0 g tri-sodium citrate (Na3C6H5O7.2H2O) and 18.0 g sodium hydrogen tartrate (NaC4O6H5.H2O), dissolve, and make to 1.0 L with deionised water.
Digest Blank Solution
Carefully add 65 mL digest acid (H2SO4) to ≈1.0 L deionised water; add 20.0 g Na2SO4 and 2.0 g anhydrous CuSO4. Dissolve and make to 1.5 L. Alter as necessary to reflect the actual catalyst used.
Nitrogen Primary Standard
1 mL contains 0.20 mg of N.
Dissolve 0.4717 g ammonium sulfate [(NH4)2SO4; previously dried at 100°C for 4 h] in digest blank solution and make volume to 500 mL.
Nitrogen Working Standards
Dispense accurately 0, 2, 4, 6, 10, 15, 20, 30, 50 and 60 mL N Primary Standard to separate 500 mL volumetric flasks and dilute to volume with digest blank solution. These solutions contain 0, 0.8, 1.6, 2.4, 4.0, 6.0, 8.0, 12.0, 20.0 and 24.0 mg N/L, equivalent to 0, 0.012, 0.024 … 0.36% N for a soil/solution ratio of 1:150.
Weigh 1.00 g of ground (<0.5 mm) air-dry soil into a 100 mL Kjeldahl digestion flask, graduated at 150 mL. Add 2.0 mL deionised water, swirl and stand for 30 min then add 2.2 g catalyst mixture (or two catalyst tablets) and 6.5 mL H2SO4 (see Note 1). Digest as described in Method 7A1. Cool, dilute to 150 mL with deionised water and shake well. After any solids have settled, or if necessary after centrifugation, take an aliquot for autoanalysis.
Set up autoanalysis equipment as shown in flow diagram (Figure 7.4), noting that instrument selections and settings should be in accord with manufacturer’s instructions. ‘Condition’ the manifold before use, make a final check on instrument settings, degas reagents if necessary, then determine N concentrations in soil digest solutions directly from relevant working standards run on commencement and as required throughout the batch of unknowns. If necessary, dilute digests containing >24 mg N/L with digest blank solution or redigest using a reduced sample size. Also use digest blank as the wash solution during auto analysis.
Report TSN (%N) on an oven-dry basis. Use the air-dry moisture to oven-dry moisture ratio to make the oven-dry conversion. Refer to Method 2A1 for guidance with regard to this soil moisture calculation.
1. Some soils can consume sufficient H2SO4 during Kjeldahl digestion to cause errors in the subsequent colorimetric determination of N (Blakemore et al. 1987). No adjustment to the volume of H2SO4 should be necessary with this method, however, at common soil N concentrations, as the boundary condition of 15% H2SO4 consumed should not be reached, even when soils are highly calcareous.
Figure 7.4. Soil total N automated colour continuous segmented flow sheet.