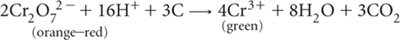
Concentrated H2SO4 is added to soil wetted with a Cr2O72– solution. Heat of dilution raises the temperature to 110–120°C, which is sufficient to induce substantial oxidation. The reaction is as follows:
Table 6.2. Summary detaÕ of method codes, technologies, method titles and notes on soÕ C tests described in this chapter.
Code |
Technology |
Test method |
Notes |
6A1 |
Wet oxidation. |
Organic C – W & B (OCW&B). |
Total OC (approximate – due to incomplete chemical reaction, subject to removal of Cl– interference). |
6B1 |
|
Total organic C – Heanes wet oxidation. |
Total OC (subject to removal of Cl– interference). |
6B2a |
Catalysed, high temperature combustion. |
Total organic C – Dumas high-temperature combustion, volumetric (no soil pretreatment). |
Measures TOC only in the absence of charcoal and carbonate interferences – otherwise yields total C. Superior technology is now available. |
6B2b |
|
Total organic C – Dumas high-temperature combustion, infrared/thermal conductivity detection (no soil pretreatment). |
Measures TOC only in the absence of charcoal and carbonate (and bicarbonate) interferences – otherwise yields total C. |
6B3 |
Physical and chemical pretreatments then catalysed, high temperature combustion. |
Total organic C – high temperature combustion furnace, infrared/thermal conductivity detection (with prior physical removal of visible charcoal and chemical removal of carbonates). |
Good estimate of TOC in soils containing visible charcoal, carbonates and bicarbonate. |
6B4a |
Infrared diffuse reflectance spectroscopy. |
Total organic C – NIR reflectance spectroscopy. |
Total OC (surrogate by correlation). |
6B4b |
Total organic C – MIR reflectance spectroscopy. |
Total OC (surrogate by correlation). |
|
6C1 |
Physical-chemical |
Particulate organic C in >53 μm dispersed soil using hexametaphosphate. |
Good estimate of soil POC. |
6D1 |
Extractable C. |
Pyrophosphate-extractable C. |
Empirical estimate. |
6E1 |
Readily labile C. |
Potassium permanganateextractable C. |
An empirical, soil C availability indicator, suited to field and lab use. |
6F1 |
Concentrated peroxide/nitric acid digestion before dry combustion of residue. |
Charcoal C by digestion to oxidise OC, before dry combustion of residue. |
Empirical: expect high recoveries at <0.1% charcoal-C. |
6G1 |
Heating to specified temperatures. |
Total organic matter, TOC and carbonate, all by loss-on-ignition. |
Low cost, approximate values. |
In the absence of interferences, the chromic ions (Cr3+) produced should be in reasonable proportion to the OC oxidised. In aqueous acidic solution, Cr3+ has absorption maxima at 450 nm and 600 nm. Since Cr2O72– does not absorb at 600 nm, the absorbance at this wavelength can be used to estimate Cr3+ and hence OC (Sims and Haby 1971; Metson et al. 1979). An advantage over the original titrimetric procedure and the colorimetric estimation of unreacted Cr2O72– solution is that accurate standardisation of the Cr2O72– solution is not required.
Since Cl– reacts to form chromyl chloride (CrO2Cl2), correction for positive Cl– interference in saline soils (>0.5% Cl) is recommended. Ferrous iron (Fe2+) may also produce high results but this possibility is usually ignored (PASS would be an exception) as air-dry soils do not normally contain significant reactive Fe2+ (Anon 1983). Elemental C (e.g. coal and charcoal) is usually little attacked by this method (except if present as very fine particles), while carbonates, even when present at high concentration (e.g. 50%) have inconsequential impacts.
Note that the sodium dichromate (Na2Cr2O7.2H2O) or chromium trioxide (CrO3) used in the procedure are powerful oxidising agents and systemic poisons. Accordingly, avoid external and internal contact and keep these chemicals away from combustible materials due to risk of fire. These are preferred to potassium dichromate (K2Cr2O7), simply for reasons of cost (Metson et al. 1979).
Reagent Water
This water should be in equilibrium with the atmosphere with respect to CO2 concentration, it should have an EC of <1.5 × 10-3 dS/m, and it should be devoid of soluble OM sufficient to affect the blank.
0.5 M Sodium Dichromate
Dissolve ≈149 g L.R. sodium dichromate (Na2Cr2O7.2H2O) in Reagent Water and make to 1.0 L. Filter through sintered glass or glass-fibre filter material to ensure the solution is free of particles prior to use.
1.0 M Chromium Trioxide (alternative to 0.5 M sodium dichromate)
Dissolve ≈100 g L.R. chromium trioxide (CrO3) in Reagent Water and make volume to 1.0 L. Filter through sintered glass or glass-fibre filter material to ensure the solution is free of particles prior to use.
Standard Sucrose Solution
1 mL contains 5 mg of C.
Dissolve 11.8745 g sucrose [(C12H22O11), previously dried for at least 24 h in a desiccator over sulfuric acid (H2SO4; 18 M)], and dilute to 1.0 L with Reagent Water in a volumetric flask.
Sulfuric Acid
Commercial sulfuric acid (H2SO4; sg. 1.84), free of suspended matter.
Prepare a series of standards for each set of analyses by dispensing 0, 1.0, 2.0, 3.0, 4.0, 5.0, ... 10.0 mL (as required) of the Standard Sucrose Solution into 250 mL conical beakers. These standards contain 0, 5, to 50 mg C, corresponding to 0–5% C for a 1.0 g soil sample and 0–25% C for a 0.2 g sample. Evaporate the dispensed sucrose solutions to dryness in an oven at not greater than 65°C then cool to room temperature.
Weigh samples of finely-ground (<0.5 mm), air-dry soil, according to the expected C content: 1.00 g for expected values of <5% C, and 0.20 g for expected values >5.0% C. Transfer soils to dry 250 mL conical beakers (all should be of similar manufacture and dimensions and located equidistance apart). To the standards and soils add 10 mL of 0.5 M sodium dichromate or 1.0 M chromium trioxide solution and swirl gently to ensure all soil particles are wetted. Wait 10 min with occasional swirling then carefully but quickly add across 10–15 sec (use gentle swirling to avoid the loss of soil and chromic acid from localised boiling) 20 mL concentrated H2SO4. Wait a further 30 min with occasional swirling then add 170 mL Reagent Water from a dispenser, stir or swirl to mix thoroughly and set aside to cool and for particles to settle. (Consistent timing across the additions of H2SO4 and dilutant water helps ensure constant reaction conditions, including heat generation.)
After cooling, centrifuge if the supernatant is not clear. Determine absorbance of the supernatant at 600 nm with Reagent Water set at zero. Construct a standard curve by plotting absorbance of the standard sucrose assays against their known contents of C (or use a microprocessor-controlled equivalent). Thereafter, dispose of spent reagents and treated soils in a safe and environmentally responsive manner, noting that Cr is a heavy metal that is both toxic and environmentally persistent.
Repeat the determination with less soil if >75% of Cr2O72– is reduced (only likely with soils of very high C content).
Report OCW&B (% C) on an oven-dry basis, allowing for the weight of sample. Use the air-dry moisture to oven-dry moisture ratio to convert to an oven-dry concentration. Refer to Method 2A1 for guidance with regard to this soil moisture calculation.
Determine water-soluble Cl (Method 5A1 or 5A2) and express results for Cl as a percentage, noting that 10 000 mg Cl/kg = 1% Cl. Also determine apparent OCW&B as described for non-saline soils. Again, repeat the determination with less soil if >75% of Cr2O72- is reduced.
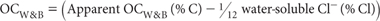
both expressed on the same soil moisture status and allowing for the weight of sample.
Report OCW&B (% C) on an oven-dry basis. Use the air-dry moisture to oven-dry moisture ratio to convert to an oven-dry concentration. Refer to Method 2A1 for guidance with regard to this soil moisture calculation.