This method, coded as 5A2 in Rayment and Higginson (1992), involves SFA, a system broadly described elsewhere (e.g. Gordon et al. 1993; Section 4120 of APHA 2005). The SFA systems herein outlined are examples based on AutoAnalyzer I/II technology and microbore technology. It is recognised that improvements in system performance are ongoing, including faster rates of analysis, lower reagent volumes, more reliability, automatic data handling, sample dilution and speedier reagent change-over. Reductions in dispersion have centred on smaller internal diameters of reaction tubing, down from 2.4 mm in the Technicon AutoAnalyzer I, to 2 mm in the AutoAnalyzer II, and to 1 mm in more modern instruments (Jodo et al. 1992).
The latest systems incorporate a better understanding of the way in which dispersion, tubing diameter and flow rate are interrelated (Gordon et al. 1993). Continuous flow instrumentation uses a peristaltic pump to draw sample extracts from the sampler into a manifold designed to mix the samples with reagents that are being continuously pumped at predetermined flow rates, and on eventually to the colorimeter detector. The analyst must be guided by the operational instructions and directions given by the manufacturer. This could require changes to the specified reagents and to the flow diagram provided for this method.
Basis of the colorimetric reaction used is the formation – in the presence of ferric ions and free thiocyanate ions – of highly coloured ferric thiocyanate in proportion to Cl– concentrations. Source of the thiocyanate ions is mercuric thiocyanate; thiocyanate ions are liberated when soluble mercuric chloride forms. Although I– and Br– are also measured along with Cl– (TA Beech, pers.comm.), it is normally assumed for natural soils that this automated ferricyanide method has no interferences of significance (APHA 1998).
Stock Ferric Nitrate Solution
Dissolve 202 g ferric nitrate (Fe(NO3)3.9H2O) in 600 mL deionised water, add 31.5 mL nitric acid (HNO3; 14 M) and dilute to 1.0 L with deionised water. Store in a dark bottle.
Dissolve 4.17 g mercuric thiocyanate [Hg(CNS)2] in about 500 mL methanol (CH3OH) and make to 1.0 L with CH3OH. Filter if necessary.
Wetting Agent – Brij 35
Shake 30 g of polyoxyethylene 23 lauryl ether (Brij 35) with 20 mL iso-propyl alcohol [propane-2-ol, (CH3)2-CH-OH] until dissolved; several hours may be required. Make to 100 mL with deionised water.
Chloride Colour Reagent
When required, combine 150 mL ferric nitrate solution, 150 mL mercuric thiocyanate solution, 1.0 mL Brij 35 wetting agent and make to 1.0 L with deionised water. Store in dark bottle but not under refrigeration.
0.75 M Nitric Acid
Dilute 54 mL nitric acid (HNO3; 14 M) to 1.0 L with deionised water then add 1.0 mL Brij 35 wetting agent.
Chloride Primary Standard
1 mL contains 2 mg of Cl.
Dissolve 3.2969 g sodium chloride (NaCl; previously dried at 105°C for 4 h) in deionised water and make volume to 1.0 L.
Chloride Working Standards
Add 1.0, 2.5, 5.0, 12.5, 25.0, 37.5, 50.0, 75.0, 100.0 and 125.0 mL of Cl Primary Standard to separate 1.0 L volumetric flasks and dilute to volume with deionised water. These solutions contain 2.0, 5.0, 10.0, 25.0, 50.0, 75.0, 100, 150, 200 and 250 mg Cl/L and for a 1:5 soil/water ratio are equivalent to 10.0, 25.0, 50.0, 125, 250, 375, 500, 750, 1000 and 1250 mg Cl/kg of air-dry soil.
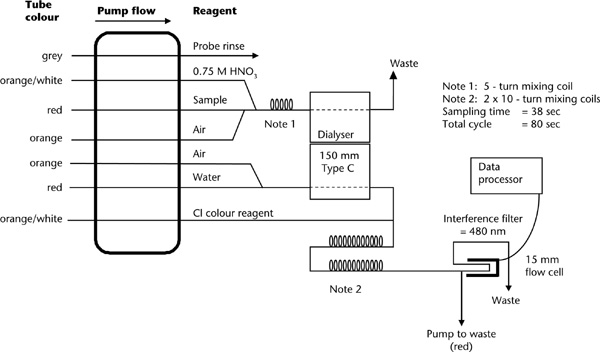
Prepare 1:5 soil/water suspensions as described in Method 3A1. Filter or centrifuge a suitable aliquot for automated SFA. Ensure components of the manifold are assembled as detailed on the relevant flow sheet (see Figure 5.2 or Figure 5.3; Figure 7.7 if both Cl– and NO3-N are required), or adapt as guided by the manufacturer. Instrument selections and settings should also be in accord with manufacturer’s instructions.
‘Condition’ the system before use by pumping reagents and relevant standard solutions. Make a final check on instrument settings, then determine Cl– concentrations in soil extracts directly from standard solutions run on commencement and as required throughout the batch of unknown soil extracts. Use deionised water as the wash solution and to dilute soil extracts of high Cl– concentrations.
Report water-soluble Cl– (mg Cl/kg) on an air-dry basis.
Figure 5.2. A continuous, segmented flow sheet (AutoAnalyzer I/II technology) for water-soluble chloride.
Figure 5.3. A micro-bore continuous flow manifold for water-soluble chloride.
Figure 7.7. A continuous, segmented flow sheet (AutoAnalyzer I/II technology) for water-soluble nitrate-N and Cl– if required.
Figure 5.2. A continuous, segmented flow sheet (AutoAnalyzer I/II technology) for water-soluble chloride.
Figure 5.3. A micro-bore continuous flow manifold for water-soluble chloride.
Figure 7.7. A continuous, segmented flow sheet (AutoAnalyzer I/II technology) for water-soluble nitrate-N and Cl– if required.